In August 2020, one Canadian biotech pharma company"FSD Pharma" received the green light from the Food and Drug Administration to design a proof-of-concept study evaluating the effects of ultra micronized palmitoylethanolamide bulk (PEA) in COVID-19 patients.
FSD Pharma filed an IND with the FDA on August 28, 2020, and was approved on September 25, 2020, to initiate a phase 2 clinical trial for the use of FSD201 to treat COVID-19, the disease caused by the SARS-CoV-2 virus.
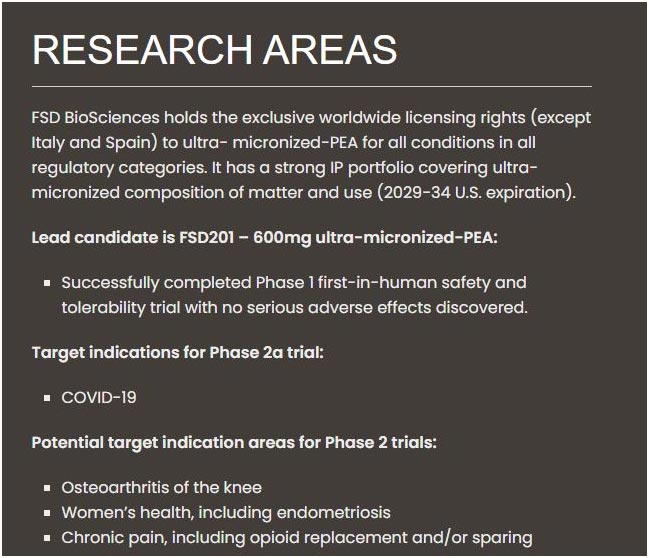
At present, FSD Pharma has Successfully completed Phase 1 first-in-human safety and tolerability trial with no serious adverse effects discovered.
The lead candidate is FSD201-600mg ultra-micronized-PEA
Target indications for phase 2a trial:
COVID-19
Potential target indication areas for Phase 2 trials:
Osteoarthritis of the knee
Women's health, including endometriosis
Chronic pain, including opioid replacement and/or sparing
We could see the following from the website of FSD Pharma: